Detecting cells in flowing liquids using TriPleX®-powered Lab-on-Chip Platform
Project I-Gene is an EU-funded HORIZON 2020 project that explores a new concept for genome surgery by laser-activation based on the Nobel-awarded CRISPR/Cas9 technique for cutting DNA. LioniX International developed an opto-fluidic device to precisely control the laser light using TriPleX®, to build a Lab-on-Chip platform for experimental confirmation of this novel idea. Our life science application specialist Dr. Arnoud Everhardt presented the work at Photonics West 2024.
Imagine yourself as a life scientist that has a revolutionary idea to cure cancer without too many side effects. You build your molecules, place them in the right cell, and now all you need to do is turn the light on and get some results to be the world’s hero. Yet, nothing happens – your flashlight’s focus falls short, and even with the help of your physicist friend who uses lenses to illuminate just the right cell, it still does not work, because the cell erratically moves away! So, what do you do? You call your rescue team at LioniX International, who immediately jump into action with a brand-new TriPleX® chip!
Modifying DNA or manipulating the genetic code of an organism in any way is a scary and incredibly powerful operation that could be done with 21st century genetics. With molecular surgery as a practical goal, there’s potential for more effective cancer treatment through secure and efficient genome editing. The spotlight is on light-induced molecular surgery, chosen for its precision in managing wavelength, power, and exposure time. Light is also a fantastic tool for the detection of (living) cells. These modalities are ideal for opto-fluidics and their common sweet spot can be reached by combining integrated photonics and microfluidics on a chip.
The combination of integrated optics with microfluidics has long been an important mission in the life sciences industry. This synergy would allow scientists to create exactly controllable environments for repeatable, precise experiments, with far less variability than true living systems. The key ingredients of such devices include microfluidic flow control to precisely bring particles where they are required to be; full control over the light, and lastly an integrated assembly in a robust package.
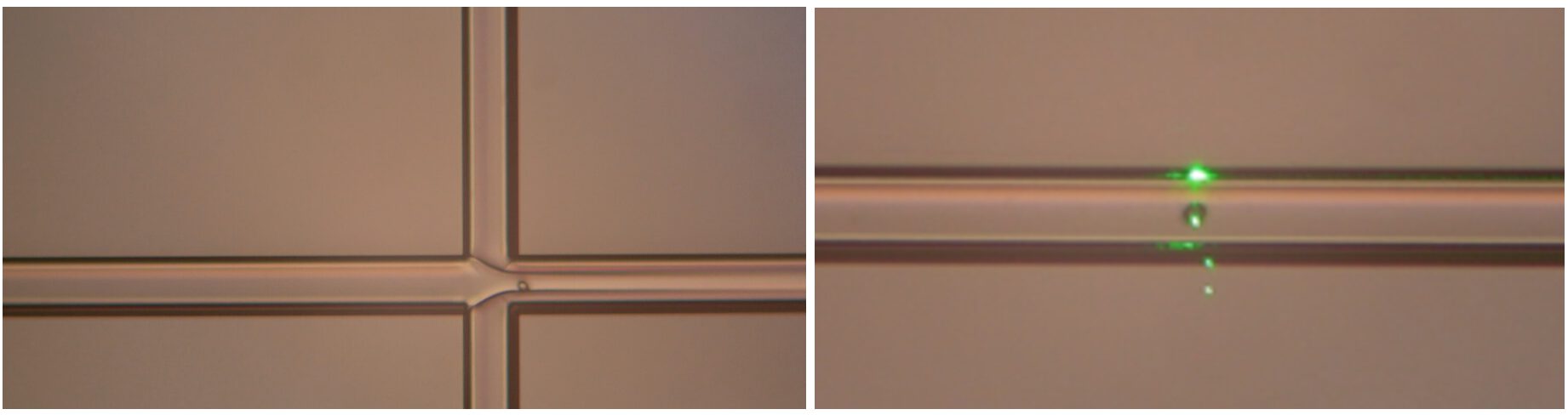
All these requirements have been successfully demonstrated. A small microfluidic flow channel, measuring 100 by 100 micrometers, has been produced inside the TriPleX® platform. Living cells are made to flow through these channels. It is not only important to have cells in the flow channels, but also to control where they are in the flow channel. This can be seen in Figure 1, where side sheath flows (a dummy flow that does not contain any cells) that visibly pushes the flow containing the cells to the center of the microfluidic channel.
Secondly, the TriPleX® waveguide platform illuminates a well-controlled section of the microfluidic flow with a known power density. The light is delivered efficiently: using green light, known for its high losses in nearly all photonic platforms, 68% of the laser light reaches the cell exposure area. High powers of several Watt, and power densities exceeding several mW/µm2, allow brilliant illumination, and in the case of molecular surgery, pass the threshold for safe DNA cutting!
Figure 1. Left: the flow focus built into the TriPleX® platform forces the cells to the center of the microfluidic channel. Right: A waveguide is brought close to the microfluidic channel and then exposes a single cell with green laser light.
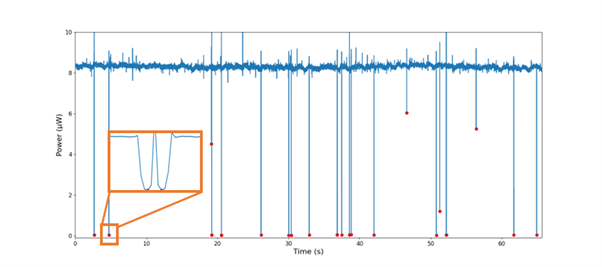
Bringing the light to the cells does not only mean you can illuminate the cells, but you could also measure what happened to the light! Placing a second waveguide across the microfluidic channel allows it to measure when the optical power drops, indicating that a cell passing by has blocked the light! In this way, an opto-fluidic ‘cell counter’ has been produced and validated to count individual cells in Figure 2.
Figure 2. The power measured on the second waveguide is usually 8 µW – until a cell flows by, dropping the power to nearly zero, demonstrating a very accurate cell counter. Even two cells flowing just after each other can be distinguished in this method, as shown by the inset.
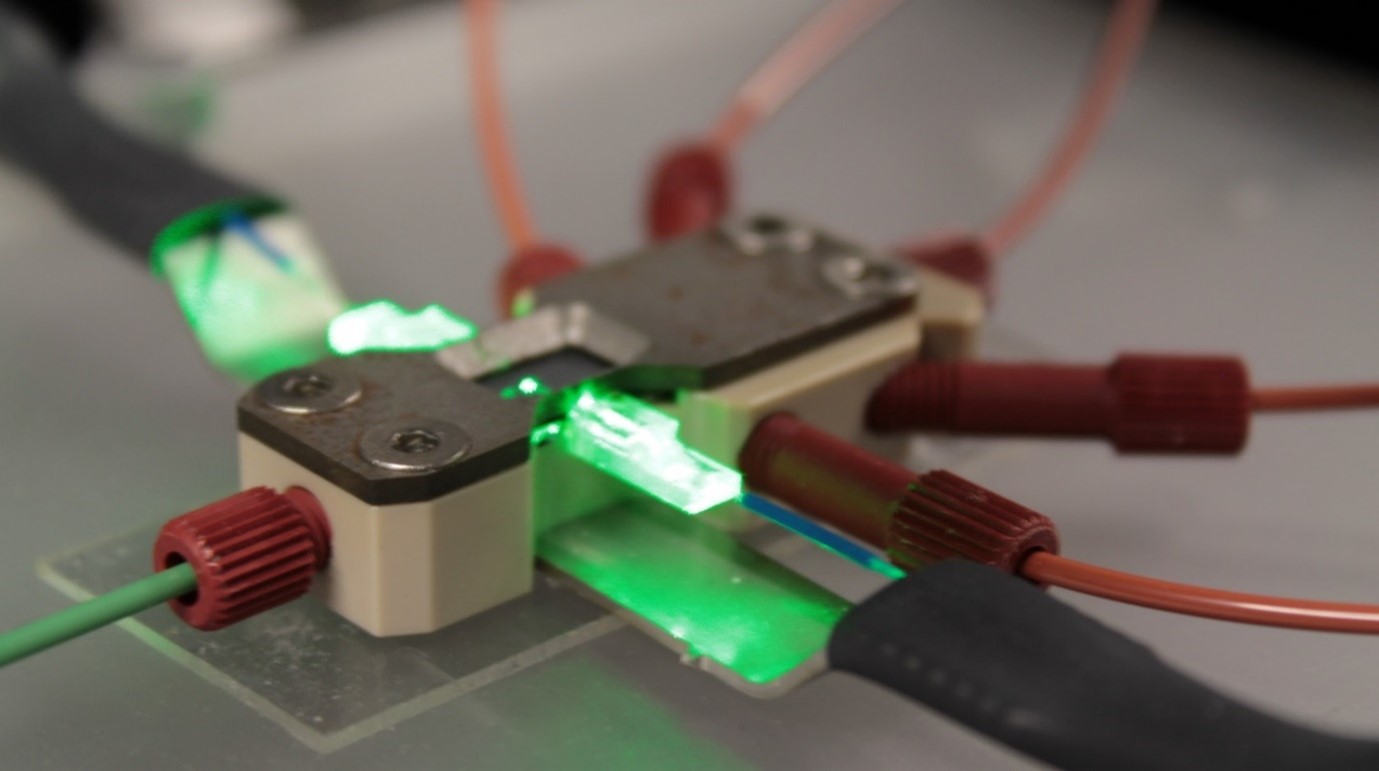
All of this does not exist as just a chip and some liquids, but needs specifically designed packaging to connect it to the outside world. LioniX International developed custom packaging for this specific need, integrating both optical fibers and microfluidic connectors for all the different microfluidic flows required.
Figure 3. Custom packaging for this specific opto-fluidic solution for cell flow, illumination, and detection.
With this, the specific needs of a life scientist have been met: achieving precise control over both the positioning of cells and light, as well as exerting influence over optical power and shape. Now, our life scientist (who in this story, as you surely remember, is you, dear reader) knows to conduct precise experiments, receive real-time feedback on the movement of cells, and potentially revolutionize new methods for curing cancer!
And what if you are not a life scientist? The concepts and results are custom and modular and can be integrated on TriPleX® with any other design of your choosing. Why not build the world’s smallest and most sensitive flow cytometer as a cheap and portable chip which not only determines the presence of particles, but also their size and optical properties in a liquid flow?
Want other examples of life science applications integrated on TriPleX®? Read on:

Arnoud Everhardt PhD (Design Engineer/Project Manager) has been active in integrated photonics since 2019 at LioniX International. His main focus is to develop different types of photonic biosensors. Several types include refractive index-based sensing using chemical probes, OCT (optical coherence tomography) for depth scanning in tissue or microfluidics, flow cytometry and here the integration of microfluidic channels into biosensing.