About Project PHOREVER:
Funded by the European Commission, project PHOREVER aims to innovate past existing imaging modalities for the most lethal human diseases. Cancer and cardiovascular disease imaging and detection devices are expensive, complex, biologically harmful in some instances. In all instances, they cannot characterize disease progress, recurrence, or resistance to therapy.
The information required to track these diseases over time is widely believed to be present within particles carried by blood: cells, vesicles, and biomolecules. As current imaging techniques operate on the macro level, they cannot detect or assess such microscopic objects.
Extracellular vesicles (EVs) are of particular interest in this application, as they bud out of cells elsewhere in the body and travel through bloodstreams to carry out waste removal and cell-to-cell communication functions. To carry out these functions, they carry disease-specific proteins (biomarkers for those diseases) on their membranes as cargo. Thus, they can provide key information about the physiological and pathological states of the organs they originate from. Such cells are typically 50 – 200 µm in radius, for which flow cytometry (FCM) is the most promising detection and characterisation method.
However, existing commercial FCM modalities can only detect particles of 150 – 200 µm radii. To address this gap, PHOREVER will develop a multi-sensing platform capable of detecting EVs with radii as small as 80 nm via light scattering. It will also calculate their concentrations in given blood samples via cytometry and verify the presence of disease biomarkers on them via fluorescence spectroscopy. Optical coherence tomography (OCT) will be used as an additional imaging modality for the verification of the light scattering FCM results.
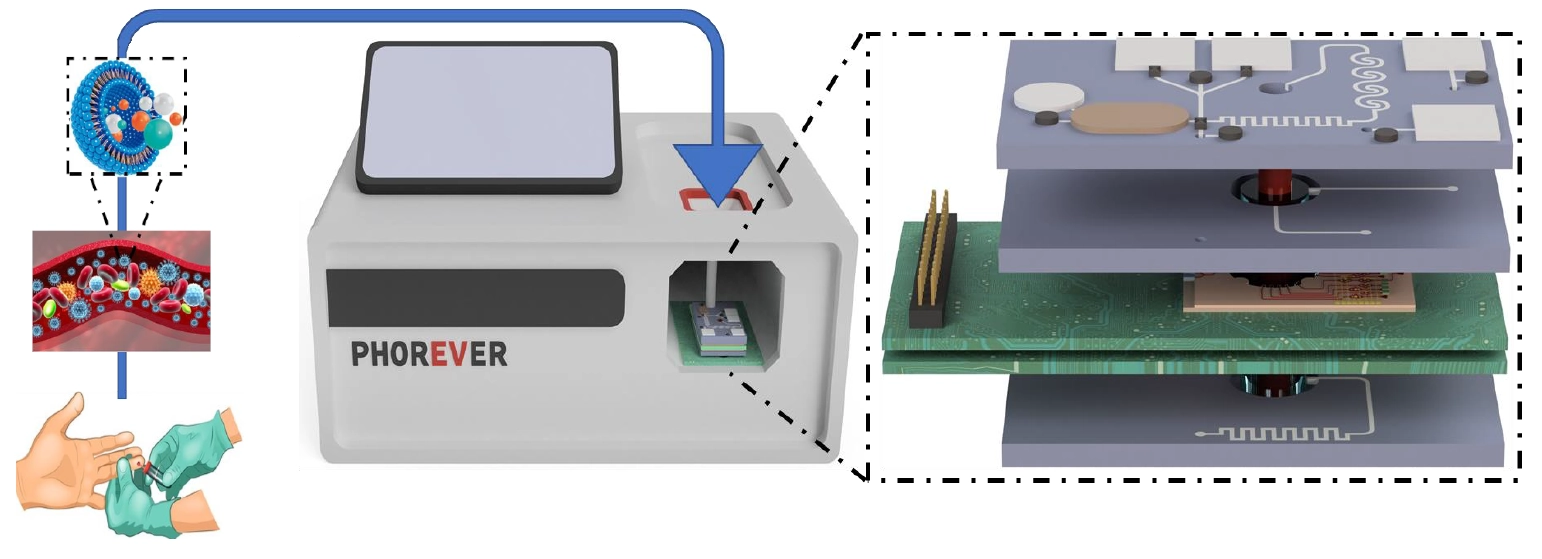
Through on-chip integration, the device will enable point-of-care diagnosis, disease progression monitoring, and therapy assessment. It will be composed of 2 photonic integrated circuits (PICs) and 3 microfluidic chips. It will be used to track pancreatic cancer progression, metastasis risk, and treatment efficacy via the correlation of EV blood concentration. It will also be used for the fast diagnosis and identification of cardiovascular stroke types through the identification of specific EV blood concentrations. Data generated for both diseases will be correlated to patient medical data using AI-powered data analysis algorithms.
Outline of project PHOREVER, featuring the microfluidic and photonic chips and a mockup of a diagnostic device utilizing them.
The Role of LioniX International:
Besides providing input in the technical management and knowledge exploitation of the project, LioniX International will design two PICs and fabricate them on our TriPleX® platform: one for FCM and fluorescence measurements, another for the OCT measurements.
The first PIC will feature an illumination waveguide for directing light from different laser diodes towards the plasma-carrying microfluidic channel, signal waveguides for collecting light scattered by the particles in the plasma towards photodiodes, and a beamsink waveguide for collecting unscattered light to prevent it from affecting measurements. The scattered light waveguides will feature filters tuned to the wavelength of the switched-on lasers, which are in turn controlled by a time-division multiplexing scheme on-chip.
After a median section for the addition of fluorescent molecules to the targeted biomarkers, a second part of the PIC will include an FCM section for the detection and measurement of EVs down to 80 nm radii. The final section of the first PIC will include a fluorescence subcircuit, which will use notch filters to block out excitation light and photomultiplier tubes (PMTs) to detect emission light from the sample. The integration of the laser diodes, PMTs, and their respective focusing polymer micro-lenses will be facilitated by the spot size converters possible on TriPleX®.
The OCT PIC will be the first of its kind in supporting the complete swept-source OCT (SS-OCT) process on-chip. Using the same microfluidic channel as the first PIC, SS-OCT will image the sensing area to collect information about the number and position of particles within the plasma. This data will serve as a ‘coherent gate’ for the data collected by the FCM modality. The PIC will use TriPleX® external cavity tunable lasers, with 100 nm sweeping span, stress-optic tuners, and sub-micron operation. Detector photodiodes will be integrated to the PIC for data collection using the spot size converters and polymer microlenses.
Further information:
To learn more about PHOREVER, please visit the project website.